Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Nitrogen nip and tuck
Anomeric amides remove nitrogen atoms from secondary amines
by Bethany Halford
May 12, 2021
| A version of this story appeared in
Volume 99, Issue 18

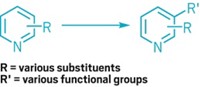
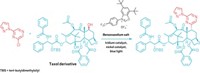
A new reaction cuts nitrogen atoms out of secondary amines with surgical precision. The transformation offers a route to strained structures, such as cyclobutanes (example shown), that can be difficult to make by other methods. It also gives chemists a quick way to explore the effect a single nitrogen atom has on a molecule’s properties by letting them compare the biological activity of drug candidates before and after the reaction.
“You can imagine that there are circumstances in which making a carbon-nitrogen bond is a lot easier than making the corresponding carbon-carbon bond,” says University of Chicago’s Mark D. Levin, who led the group that developed the new reaction. With this transformation, he says, chemists can take advantage of established reactions for making C–N bonds and, with one more step, forge those challenging C–C bonds (Nature 2021, DOI: 10.1038/s41586-021-03448-9).
The key reagent in the nitrogen-extracting reaction is an anomeric amide, which features a nitrogen bound to two oxygens. The chemists think that the secondary amine attacks the anomeric amide, which ultimately generates an isodiazene intermediate. This intermediate releases dinitrogen via a radical process, generating radicals at the two carbons that were attached to the nitrogen of the secondary amines. These radicals then combine to form a C–C bond. Levin points out that for the nitrogen nipping to work, at least one of the carbon radicals needs to be adjacent to a stabilizing group, such as an aromatic ring.
Tim Cernak, an organic chemist at the University of Michigan, says his jaw dropped when he first saw Levin’s paper. “If you drew this reaction for me last week, I’d say it’s impossible,” he says in an email. “It just wouldn’t be conceivable to walk up to the chalkboard and rub out a nitrogen atom on your molecule before this.” What’s more, Cernak says, this new reaction will likely be one of the early landmarks in the field of molecular editing, where chemists insert, delete, or replace a single atom in a molecule.
Levin says he is working on commercializing the anomeric amide reagent the group used. In the meantime, he says the compound is easy to make for chemists who want to try the reaction for themselves.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter