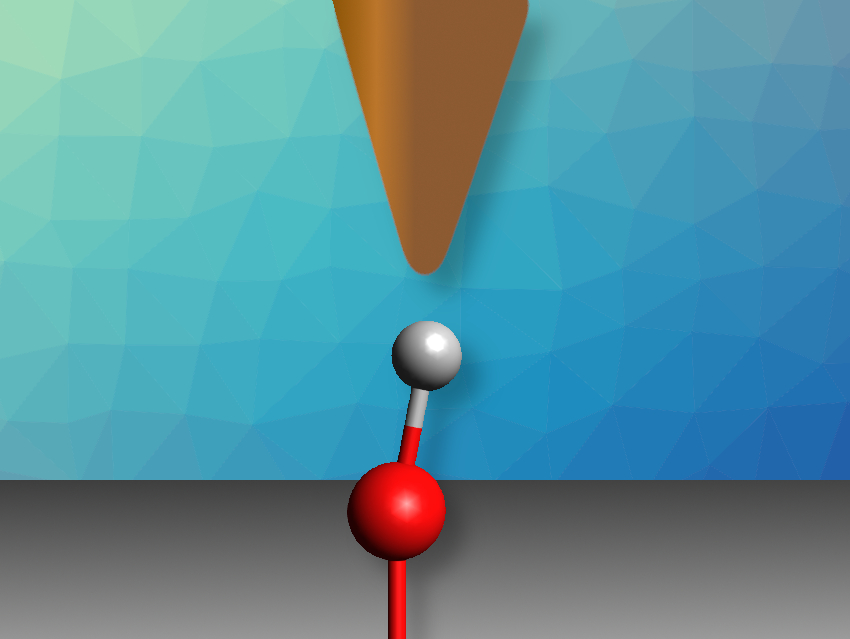
The acidity of a chemical species can be described by its proton affinity and its pKa value. The state of protonation of surfaces is important, e.g., in catalysis, materials science, or biochemistry. However, the acidity of individual sites on a surface is difficult to assess in contrast to molecules—only an average value could be measured so far. The acidity of individual sites can be predicted using computational methods, but until now, there had been no experimental approach to verifying these predictions.
Ulrike Diebold, TU Wien (Vienna University of Technology), Austria, and colleagues have experimentally assessed the acidity of individual hydroxyl groups on the In2O3(111) surface, an oxide with four different types of surface oxygen atom that might be protonated. The team probed the strength of hydrogen bonds formed by these hydroxyl groups using the OH-functionalized tip of a non-contact atomic force microscope (AFM). The OH group on the AFM tip forms a hydrogen bond with one OH group on the surface. The proton affinity of the surface OH group can then be calculated from the strength of this hydrogen bond, which the team determined by varying the distance between the tip and the surface and measuring how this changes the force between them.
By comparing the results of the AFM measurements to the known proton affinities of probe molecules, the team was able to determine the proton affinities of the different surface sites of In2O3. They found that the results agreed quantitatively with the results of density functional theory (DFT) calculations. The method could also be extended to hydroxylated titanium dioxide and zirconium oxide.
- Direct assessment of the acidity of individual surface hydroxyls,
Margareta Wagner, Bernd Meyer, Martin Setvin, Michael Schmid, Ulrike Diebold,
Nature 2021.
https://doi.org/10.1038/s41586-021-03432-3